Research
Our Research
Our Purpose
Although many factors involved in seed development and seed sizes/numbers have been identified, the precise mechanisms of how plants accomplish seed development and control these seed traits are largely unknown. Using Arabidopsis thaliana, soybean, and the liverwort Marchantia polymorpha, we are investigating the molecular mechanisms, cellular dynamics, and evolution of land plant sexual reproduction, especially focusing on stages from fertilization to early seed/embryo development.
(i) What are the molecular and cellular mechanisms that control precise fertilization processes in plants?
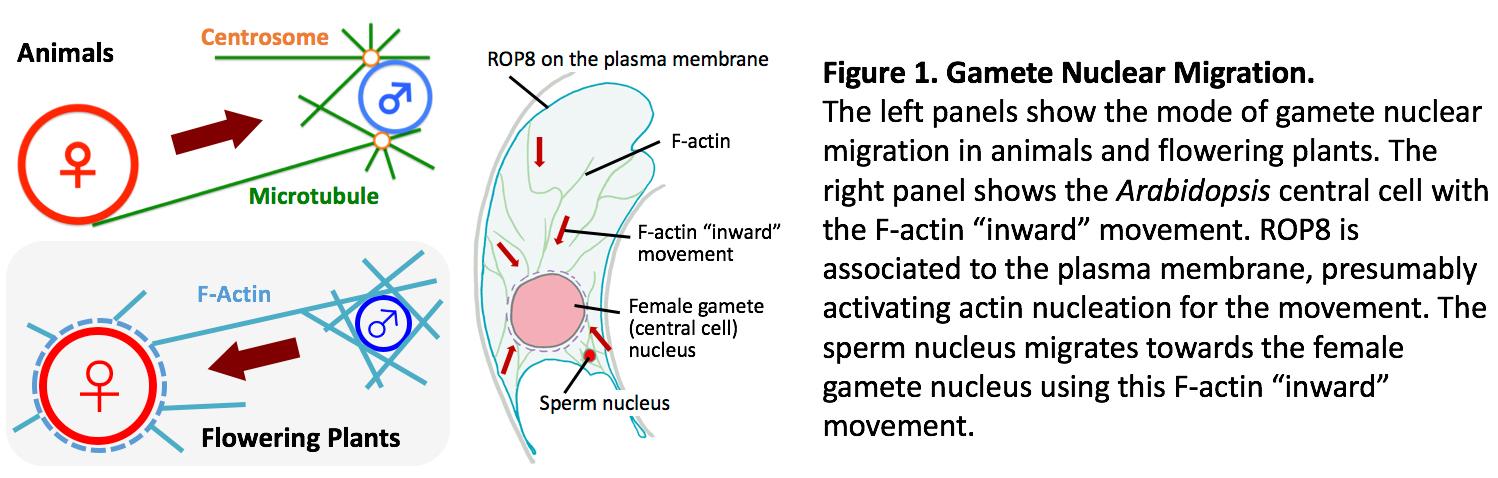
Unlike in animals, molecular and cellular mechanisms of fertilization processes in flowering plants still remain largely unknown. This is mainly due to the inaccessibility of fertilization processes in flowering plants to direct observation. We have established a method to allow real-time live-cell imaging of seed development including fertilization process in Arabidopsis thaliana. Interestingly, the process for gamete nuclear migration in flowering plants is quite different compared to that in most animals; in plants, F-actin rather than microtubules is responsible for sperm nuclear migration. The constant F-actin movement towards the center of the cell, where the female gamete nucleus is located, takes place in one of the female gamete cells, the central cell. Except for the involvement of a Rac/Rop family of small GTPases, how this constant “inward’ movement of F-actin in the gamete cell is controlled remains completely unknown. Using forward and reverse genetics and real-time live-cell imaging, we want to understand how plants control such orchestrated fertilization processes. The knowledge that we obtain from this project will provide the molecular and cellular mechanisms of how flowering plants utilize and regulate F-actin dynamics for gamete nuclear migration.
(ii) How do plants regulate unique endosperm development?
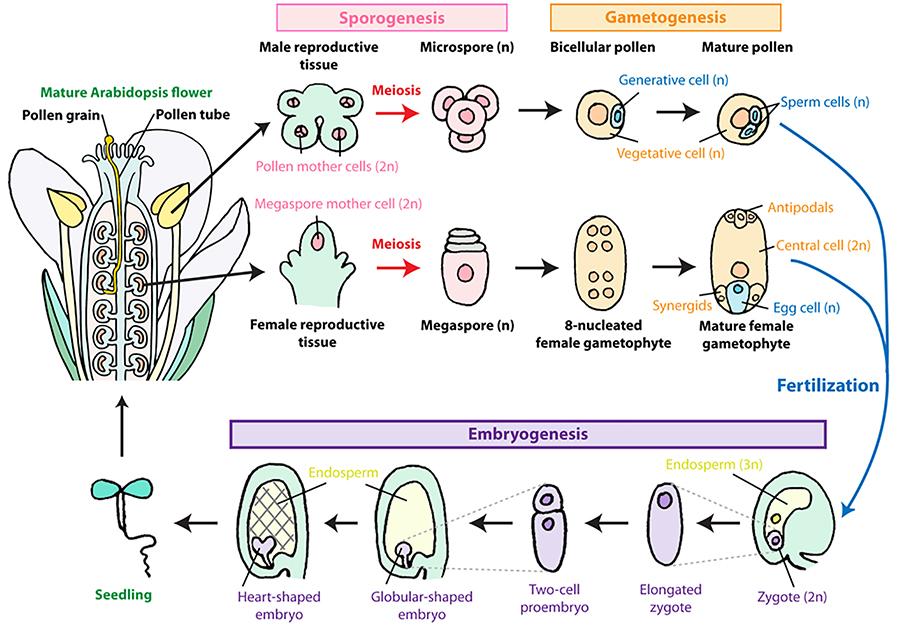
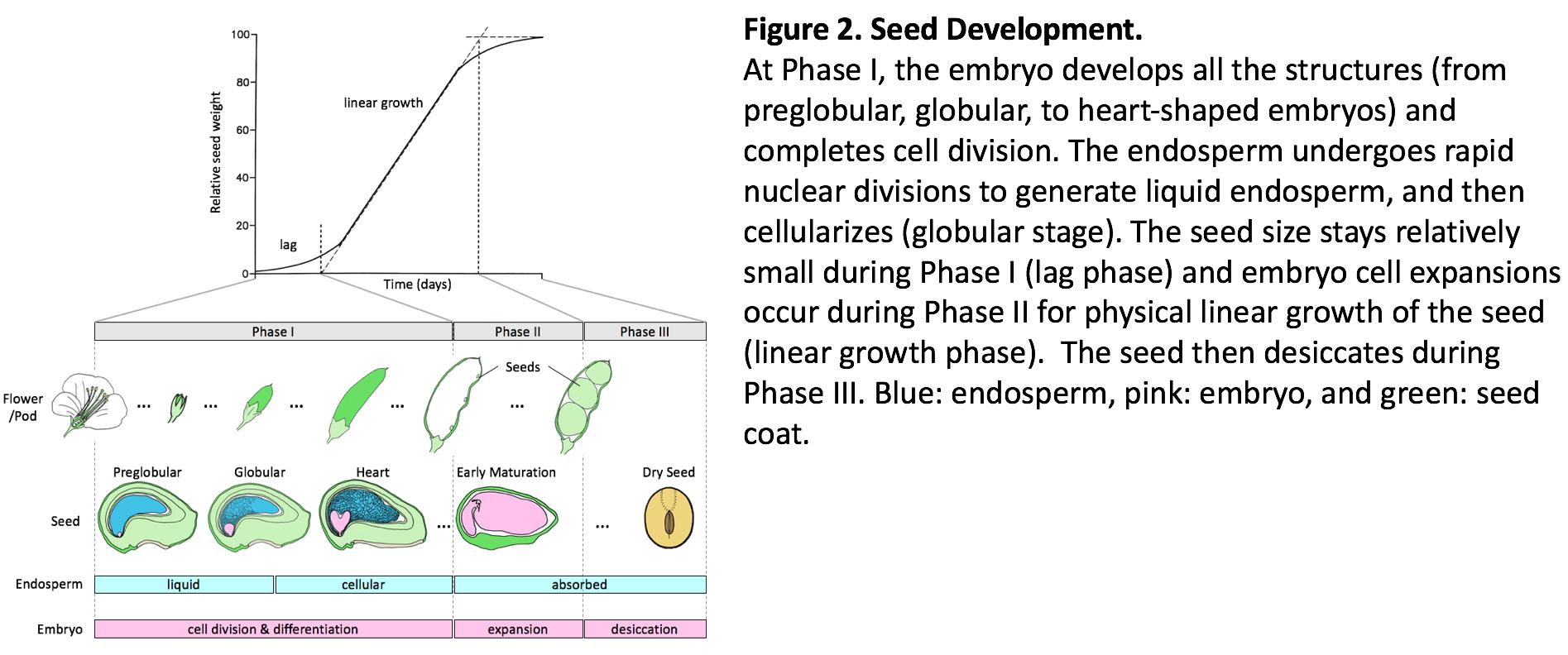
After a double fertilization unique to flowering plants, the zygote divides to form a globular-shaped embryo. The embryo keeps dividing and cotyledon(s) emerge during axis formation, followed by more cell divisions to complete the embryo structure. Fertilization of the other cell (the central cell) triggers rapid nuclear divisions without cytokinesis, generating a multinuclear liquid endosperm. The rapid nuclear divisions continue until the globular stage, and the endosperm cell then undergoes cytokinesis (cellularization). All the seed structures are formed and cell divisions in the cotyledons and endosperm are completed during this early development phase. Cell numbers and seed growth rates are correlated across genotypes of soybean and maize, suggesting that the number of cell divisions set during the lag phase is under genetic control and is one of the determining factors of seed growth rate during the subsequent linear growth phase. The fact that genetic differences in seed growth rate in soybean are maintained when growing cotyledons in vitro with excess nutrient levels further supports the idea that the “metabolic machinery” potential of the seed, partly dependent on the cell number, is determined early on during the early phase of seed development. A better understanding of the processes involved in embryo and endosperm cell division during this early phase and its duration, and how these processes are influenced by environmental conditions, is critical to build robust mechanistic models that describe seed growth and yield of grain crops. Using Arabidopsis and soybean, we are dissecting out how early endosperm development is controlled from a cytoskeleton dynamics point of view. We are also integrating environmental factors, such as the temperature, to understand the relationships among genotypes, environments, and phenotypes of seed size and yield.
(iii) How did plants evolve sexual reproduction during land plant evolution?
In mostanimals, the paternally inherited centrosome organizes microtubules to generate a sperm-aster, pulling the egg pronucleus towards the male pronucleus to complete fertilization. In early diverging plants, they no longer utilize the centrosome in somatic cells; however, the blepharoplast, analogous to the centrosome, is established during male germline development, and the microtubule still plays an important role in sperm nuclear migration in the fern, Marsilea. Flowering plants, on the other hand, have established the F-actin-based gamete nuclear migration system. Interestingly, Marsilea shows the gamete nuclear migration (male towards female), but at the same time microtubules still play a role in this process. This observation suggests that land plants gradually modified the mode of gamete nuclear migration and performed a functional exchange between the microtubule and F-actin during evolution. Investigation of molecular and cellular mechanisms controlling gamete nuclear migration in land plants from bryophytes to ferns to flowering plants will shed light on how land plants evolved their cytoskeleton dynamics and usage.
Marchantia polymorpha is a liverwort, considered to be the earliest diverging land plant and its genome has been sequenced. More importantly, all genetic tools such as Agrobacterium-mediated transformation, homologous recombination, as well as CRISPR/Cas9 system, are available. As the initial step, we will establish real-time live-cell imaging of Marchantia fertilization and embryo development, enabling further understanding of the dynamics and evolution of sexual reproduction in land plants.
A Marchantia thallus (Shohei Yamaoka, Kyoto University)

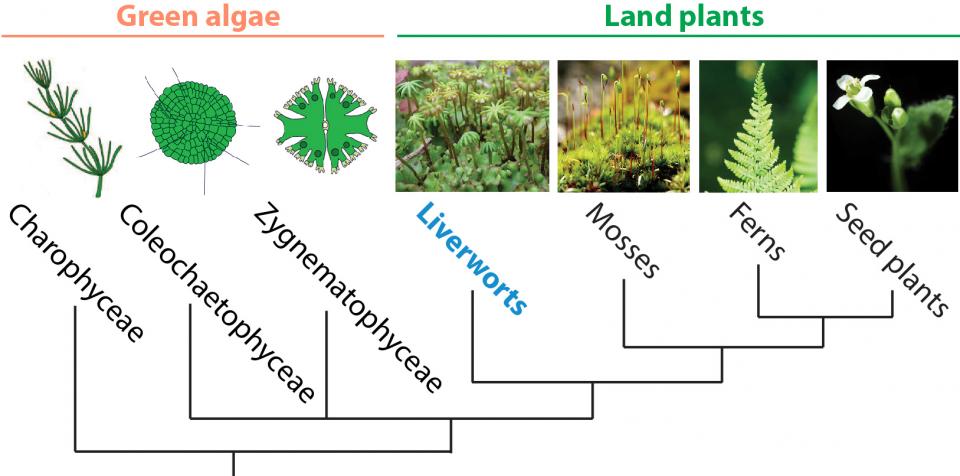
Green plant phylogenetic tree